Is So4-2 Polar Or Nonpolar
SO42- Polar or Nonpolar – Sulfate Ion Polarity Explained
SO42- is a chemical formula for Sulfate ion; it comprises one Sulfur Atom and four oxygen atoms. It besides has a -2 accuse because of the additional electrons it accepts to attain this structure. This blog postal service will help yous understand if this ion is polar or nonpolar, although a -2 accuse might confuse you. It is meliorate to understand the polarity by the Lewis Construction, Shape and Net Dipole moment of the molecule.
What is polarity?
When we talk most polarity, it is basically a phenomenon due to the separation of electrical charges in the given molecule, which leads to an electric dipole in the molecule. Polar molecules consist of polar bonds, and that is because of a difference in electronegativity betwixt the atoms forming bonds. However, polarity also depends upon the shape of the molecule and the lone pairs.
SO42- Lewis Structure and Shape
If you look at the Lewis Construction of SO42- ion, information technology is quite simple. The sulfur atom is in the centre, forming double bonds with two Oxygen atoms and single bonds with the remaining two Oxygen atoms. The shape of the molecule is tetrahedral, and it is quite symmetric likewise. Besides that, the negative charges are due to the single bonds with Oxygen atoms.
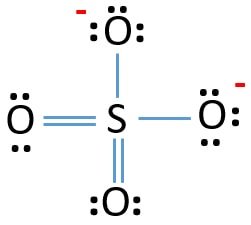
As mentioned higher up, solitary pairs also contribute to the molecule'south polarity; there are no lone pairs of electrons on the central Sulfur atom.
Electronegativity and Bail Nature
One time you know the shape and other details of the molecule, information technology is crucial to find out the bail nature in the molecule. Sulfur's electronegativity value is 2.six, and for Oxygen, it is 3.44; hence when you summate the difference in electronegativities, it is higher than 0.iv.
Every bit the difference is higher than 0.4, the bonds between Sulfur and Oxygen are polar. Meaning there will be a dipole moment in the direction of the Oxygen atom.
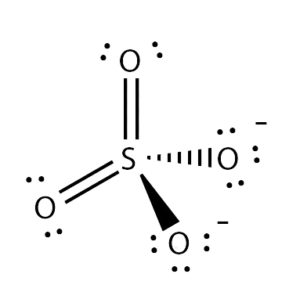
Net Dipole Moment in SO42-
The bonds in this molecule are polar, and therefore at that place will be a dipole moment from Sulfur to Oxygen atoms. However, if you await at its shape, all the Oxygen atoms are arranged as far as possible, which leads to nullifying each other's dipole moment. Such arrangement cancels the opposite dipoles, resulting in a zero net dipole moment.
Thus, SO42- is a nonpolar ion.
Why is SO42- considered a nonpolar ion?
A lot of people don't believe that this molecule is nonpolar as it can dissolve in polar solvents. It can deliquesce in polar solvents considering of the negative charges nowadays on the two Oxygen atoms. There are no poles formed in this molecule as the net dipole moment in the molecule is zippo due to the arrangement of Oxygen atoms. So although there is no polarity in the molecule, it can dissolve in polar solvents.
Last Remarks
The sulfate ion or SO42- is nonpolar because there is zero net dipole in the molecule. The symmetric arrangement of Oxygen atoms nullifies the dipole moments in the molecule, which makes it a nonpolar ion. I hope this blog post helps y'all understand the polarity of Sulfate ion with ease.
Is So4-2 Polar Or Nonpolar,
Source: https://geometryofmolecules.com/so42-polar-or-nonpolar/#:~:text=The%20sulfate%20ion%20or%20SO42,net%20dipole%20in%20the%20molecule.
Posted by: bergerfaids2000.blogspot.com


0 Response to "Is So4-2 Polar Or Nonpolar"
Post a Comment